Development of bioreactors for the biotech industry
The market for cultivation and fermentation equipment expressing biologicals is huge, growing and profitable. The biological products life time is very long and the market is slow to react to changes and innovation.
The industry is within several revolutions:
- Continuous Processing - will drive manufacturing cost further down on biological products. Continuous Processing offer 1-2 month cultivation time compared to conventional batch 5-6 days. Saving both time in starting fewer batch processes, reduce contamination risks. Much higher cell density during cultivation will save space and eventual investments required in facilities.
- Open-System to Closed-Systems – converting from flat stacked trays systems to high cell density packed-bed SUBs will drive the manufacturing cost of stem cells for therapeutic treatment and Personalised Medicine down. One significant benefit is eliminating the sterile room saving both investment of 10 k€/m2 and the 15 k€ cost/week operating a 100 m2 sterile lab for Open-Systems. Introducing Closed-Systems will allow clean room in hospital and others to introduce the Personalised Medicine concept. Cost of stem cells today range 30-40 k€/g and desperately need to be lowered significant before the expected revolution of therapeutic treatments will occur.
The present cell cultivation technology is some 35 years old and in many ways now highly optimised. The processes dominating the market is classic batch cultivation over a period of 5 - 7 days increasing the cell density from app. 0.05 mio/ml to 10.0 mio/ml. The process equipment is mostly made of glass and stainless steel based on only temperature, dissolved Oxygen and pH sensors.
The driving force for the development of Single-Use-Bioreactor (SUB) technology was the needs of the biopharma business. Over the last decade it has become clear that the old paradigm of block buster drugs is over. The future health care market will focus on therapeutic treatments for a larger number of smaller patient groups. Flexibility and significantly reduced development and manufacturing cost is the driving factor for the development of the equipment business.
The Atlantis FP6 and HESUB FP7 research projects inspired Stobbe Group to develop new business opportunities through the following daughter companies:
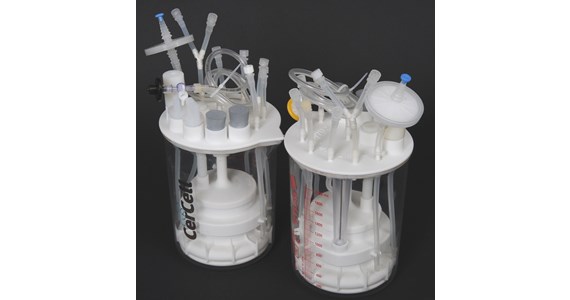
CerCell - www.cercell.com - developed a range of single-use product lines:
- CellVessel - features a series of configurable SUBs (Single-Use-Bioreactors) substituting the present glass/steel STR (Stirred-Tank-Reactors) though maintaining the batch and fed-batch processes presently in use
- CellRider - modified CellVessel able to easily accommodate end-user semi-continuous perfusion system
- BactoVessel - is the world first configurable SUF (Single-Use-Fermenter) for microbial fermentation for batch operation
- CellTumbler - a simple and reasonable cost wave-bag system
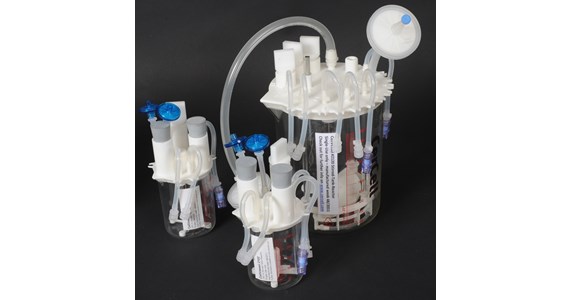
PerfuseCell - www.perfusecell.com - developed a range ready-to-use single-use products:
- CellMembra - semi-continuous perfusion system for cell retention operating at 25-100 mio /cells/ml.
- CellRetention - semi-continuous perfusion system for cell retention operating at 25-100 mio /cells/ml.
ProlifeCell - www.prolifecell.com - developed a range scaffold based SUB products:
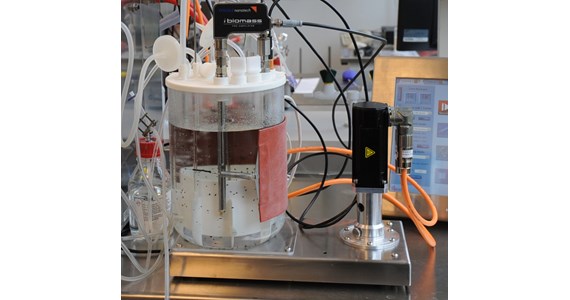
- CerCore / CellCore based HE-SUB's for continuous proliferation of stem cells operating at cell density levels of 50 mio cells/ml with continuous harvest of stem cells for therapeutic treatments or for mass production of stem cells for human organ 3D printing.
- CerCore / CellCore scaffold based CP-SUB's for continuous cultivation of mammalian cells operating at cell density levels of >150 mio cells/ml expressing antibodies. As the living conditions for the cells are more physiologically correct, the specific productivity is increased by as much as 40 % (compared to batch). Operation time of more than a month with constant high cell viability is documented so far.
- Melpomene in CP-SUB integrated diaphragm pumps
PumpCell – www.pumpcell.com – development, manufacturing and marketing of Single-Use-Pumps
- Mnemosyne integrated diaphragm pumps
- Euterpe stand-alone diaphragm pumps
Cronus-PCS – www.cronus-pcs.com – development, manufacturing and marketing of Process-Control-System units
- Clotho, Lachesis, Eirene software and Metis Drive Unit's for control of Clio, Thalia, Melpomene Single-Use-Pumps as used in CellMembra, CellRetention and CerCore SUBs
- Atropos unit for control of Euterpe stand-alone Single-Use-Pumps
CerCell, PerfuseCell, ProlifeCell, Cronus-PCS, PumpCell are all 5 part of Stobbe Group - Per Stobbe - 2019