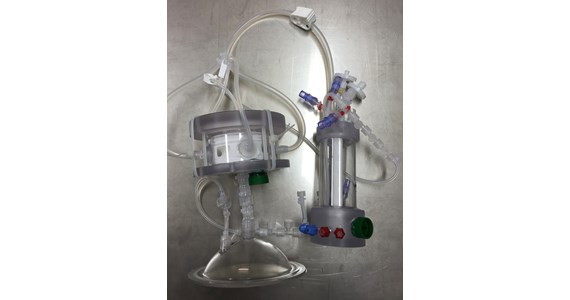
HESUB Project
The project combines several individual technologies from previous FP projects into one product that is capable of producing enough stem cells for one therapeutic treatment per day per unit. The HESUB product concept is a Single-Use-Bioreactor, which integrates a nanofibre porous scaffold optimised for the proliferation of mammalian cells and a sensor package that measures a range of key parameters, and which provides cost-efficient production of human stem cells for therapeutic treatment of a range of diseases including heart disease, diabetes, neurodegenerative diseases, musculoskeletal disorders, spinal cord injury, stroke and autoimmune diseases.
Health.2013.0-1 Innovation 2 call is an initiative designed to support SME´s efforts towards the translation of FP6 and FP7 program results into innovative and commercial products for various applications to improved human health. HESUB project gathers three innovative mature technologies issued with different horizons to create a new synergic product addressing this challenge: a new perfusion Single-Use-Bioreactor (SUB) with 3D Electrospun-Nano-Fibre Scaffold (ENF) culture support and integrated Single-Use-Sensors (SUS) combines in a Process-Control-System (PCS) vastly expanded for todays standard. The Proof-of-Concept (PoC) of the new bioreactor focuses first on cell therapeutic application to Muscular Dystrophies (MD) including Duchenne Muscular Dystrophy (DMD). For DMD treatment as approach in a previous European project, MYOAMP, injection of adult myoblasts has led to the development of new muscle fibers, but several limitations have been identified, such as poor cell survival and limited migratory ability and limited injectable cell numbers. As an alternative to myoblasts, myogenic stem cells are preferable for therapeutic applications due to their capacity for self-renewal and myogenic differentiation potential. Further the previous FP6 and FP7 European projects; RELIVER, BioComet, Atlantis are a part of HESUB.
5 European partners covering a broad spectrum of technologies and areas of expertise with the purpose of mutually developing improved and compact bioreactors for mass production of stem cells.
Approaches in muscular dystrophy treatment are multiple nowadays; stem cell-based replacement therapy is one of these. Other methods targeting at gene level, e.g. adeno-associated virus treatment correcting mal-functioning dystrophin (exon-skipping) for DMD treatment, have become highly promising. Stem cell-based therapy is viewed today as a solution for these patients or as a complementary treatment for instance in the case of insufficient precursor cell pool in the patient. Large-scale stem cell production is a key for therapy today.
Partners:
- Stobbe Tech A/S, Denmark – stobbe.com
- Electrospinning Company Ltd – England - electrospinning.co.uk
- 3Hbiomedical AB – Sweden - 3hbiomedical.com
- PreSens Precision Sensing GmbH – Germany - PreSens.de
- Royal Institute of Technology – Sweden - biotech.kth.se
Title: “Boosting the translation of health research projects’ results into innovative applications for health”
The project was partly sponsored by the EU commission under FP7 and started May 2014 and lasted 36 month.
More info around CerCore is to find at ProlifeCell - a part of Stobbe Group